1. Introduction
Bread is a staple food product in many countries and its consumption ensures enough amounts of carbohydrates for human nutrition (1). Wheat bread (WB) prepared from refined flour lacks complex carbohydrates, and its structure quickly becomes hard and not acceptable for consumers during storage, especially when gluten is weak or amylase activity in flour is high (2, 3). Scalded flour (or scald) refers to the baking dough prepared with hot water (usually 95–98°C). It is popular in Nordic European countries due to improvements in the sensory characteristics and staling process of wheat bread crumb (4). Scalding advantages encompasses the reduction of enzyme activity and non-desirable microorganisms present in flours, the increase in the concentration of extractable saccharides, and the increase of the sweet taste of bread without the addition of saccharose (5–9). This technology is used at the artisanal and industrial levels but has limited application because of the requirement of hot water and its associated economic implications (5, 10). Moreover, our previous studies showed that, on the one hand, there are many advantages of adding scalded flour to the main bread formula, however, on the other hand, it can also lead to higher acrylamide (AA) concentration in the final bread (5, 6, 9). AA is present in heat-processed foods (e.g., potatoes, rice, cereal products, fruits, vegetables, meat, fish, nuts, cocoa-based products, and coffee) at various concentrations (5, 6, 11–26). Acrylamide is a Group 2A-probable human carcinogen (27), therefore the development and application of mitigation strategies are very important, especially in popular food products such as bread (28). The Maillard reaction is primarily responsible for the generation of AA, which involves condensation of reducing sugars with free amino acid asparagine, further dehydration with the Schiff base, rearrangement to the Amadori compounds or formation of azomethine, and deamination of 3-aminopropionamide (26, 29). AA is also produced when acrylic acid, which is formed from acrolein or during Maillard reaction from aspartic acid, interacts with ammonia, produced during protein breakdown (30). It was reported that AA concentration varies in cereal-based products, and these differences are related to factors such as the content of precursors (free asparagine, glucose, fructose, and maltose), the food matrix, moisture content, and the type and duration of the thermal process (baking) (31). The strategies for reducing AA in bread were reported in various studies and mainly relate to ingredients (usage of asparaginase, polyvalent cations, antioxidants, ammonium salts, and glycine; reduction of free asparagine and reducing sugars via crop’s type, harvest, and storage conditions) and processing conditions (water activity, usage of yeast or sourdough, baking time, and temperature) (29). According to the European Food Safety Authorization (EFSA) scientific opinion, coffee and its substitutes contain the highest level of AA but the category “potato fried product” is the largest contributor to total dietary exposure to acrylamide (11). EFSA has estimated the acrylamide dietary exposure of 0.5–1.9 μg/kg b.w. per day for children, and 0.4–0.9 μg/kg b.w. per day for adults and the elderly (11), while the Joint FAO/WHO Expert Committee on Food Additives (JECFA) has reported average and high dietary acrylamide exposure of 1 and 4 μg/kg/day, respectively (29). Moreover, the FoodDrinkEurope ‘Acrylamide Toolbox’ provides the industry with scientific knowledge of AA formation and its prevention and reduction in foods (32). So far, there is no safe lowest label for acrylamide (33), although this compound concentration should be as low as reasonably achievable (ALARA) (34). The European Union Regulation Commission (EU) 2017/2158 has set the benchmark levels of 50, 100, 350, and 800 μg/kg for soft bread (wheat-based), soft bread (other than wheat), crispbread, and gingerbread, respectively (34). Therefore, taking into consideration that during flour scalding higher concentrations of fermentable sugars can be obtained and these changes can lead to an elevated Maillard reaction (5), innovative solutions should be sought and integrated, especially with regard to wheat bread manufacture – when sourdough fermentation with lactic acid bacteria (LAB) is not employed as a mean to avoid acrylamide formation in the end product.
Psyllium (Plantago ovata) plant grows in most parts of the world (15, 16) and its health benefits are widely recognized (35–43). The soluble and insoluble dietary fiber of psyllium husk possesses very good gel-forming and high water absorption ability capacities (44, 45). The major compounds of psyllium husk are pectin, cellulose, gum, lignin, and mucilage (46). Psyllium husk arabinoxylans consist of various monosaccharides as well as hydroxyl groups, which are responsible for water absorption and psyllium husk gel properties (36, 39, 40, 43, 45, 47–49).
Psyllium husk is a well-known ingredient for gluten-free bread production but the incorporation of this hydrocolloid changes the organoleptic and technological characteristics of bread (35, 50–53). However, studies on the application of psyllium husk in white bread are scarce (1, 54–56), and there are no data reporting the influence of psyllium husk on acrylamide formation in wheat bread. The main hypothesis of this study lies in the fact that psyllium husk gel (PHG) can be used as an alternative and more sustainable solution to change the scalded flour technology toward safer wheat bread. Therefore, this study aimed to evaluate the influence of different quantities (5, 10, 15, 20, and 25%) of psyllium husk gel on wheat bread quality (porosity, specific volume, mass loss after baking, shape retention coefficient, crust and crumb color coordinates, overall acceptability, and bread crumb hardness during storage) and safety characteristic (acrylamide content). Psyllium husk gel was obtained by mixing psyllium husk powder with 30°C water. Moreover, sugar (fructose, glucose, sucrose, and maltose) content in dough and bread was determined in order to better understand the changes in Maillard reaction precursors during the technological process and their influence on acrylamide formation in produced bread.
2. Materials and methods
2.1. Materials used for breadmaking
Wheat flour (type 550D, falling number of 350 s, 27% gluten, and 0.68% ash) obtained from Kauno Grudai Ltd. mill (Kaunas, Lithuania) was used for wheat breadmaking. The wheat bread samples were prepared without and with the addition of psyllium husk gel in amounts of 5, 10, 15, 20, and 25%. Psyllium husk (pure Plantago ovata in powder form; 738 kJ/176 kcal per 100 g) was obtained from JSC “Acorus Calamus” (Svencionys, Lithuania). Psyllium husk gel was prepared by mixing 100 g of psyllium husk powder with 800 mL of (30 ± 2°C) water.
2.2. Breadmaking
The bread formula consisted of 1.0 kg of wheat flour, 1.5% salt, 3% instant yeast, and 1,000 mL water (control bread). Control bread samples were prepared in the absence of psyllium husk gel. The tested dough and bread sample groups were prepared by adding 5, 10, 15, 20, and 25% (from the total flour content) of psyllium husk gel to the main recipe. Six groups of dough and bread samples were produced and examined in total. In a dough mixer (KitchenAid Artisan, Ohio, USA), the dough was blended for 2 min at a low speed, followed by 6 min at a high speed. After that, the dough was allowed to rest for 10 min at 22 ± 2°C. Following that, the dough was formed into 350 g loaves and proofed for 60 min at 30 ± 2°C and 80% relative humidity. The bread was baked for 25 min in a deck oven (EKA, Borgoricco PD, Italy) at 220°C. Figure 1 depicts a schematic illustration of the experimental design.
Figure 1. Schematic representation of the experimental design.
2.3. Evaluation of the psyllium husk gel and bread dough parameters
Color parameters of the psyllium husks gel and dough samples were evaluated using a CIE L*a*b* system (CromaMeter CR-400, Konica Minolta, Tokyo, Japan), where L* is brightness, a* is redness, and b* is yellowness, and, thus, −L* is darkness, −a* is greenness and-b* is blueness (57).
For sugar detection in dough, a 2 mg/mL standard solution of a sugar mixture (fructose, sucrose glucose, and maltose) was used. Analysis was performed with high-performance liquid chromatography (HPLC) and evaporative-light scattering detector (ELSD) LTII (Shimadzu Corp., Kyoto, Japan). The analysis was carried out in accordance with Klupsaite et al. (5). Supplementary File S1 has a full description of the sample preparation and procedure.
2.4. Evaluation of the bread quality characteristics
Bread quality characteristics were evaluated after 12 h of cooling at (22 ± 2°C). Overall acceptability of bread was carried out by 10 trained judges according to ISO 8586:2012 method (58) using a 10 score Likert scale ranging from 10 (extremely like) to 0 (extremely dislike). Additional information is given in Supplementary File S1.
Bread crumb porosity was evaluated by the Zuravliov method (LST 1442:1996). To achieve an average porosity, bread was cross-sectioned, removing the crust and crumb to form three cylinders from three distinct locations, which were then measured using the Lithuanian standard procedure (LST 1442:1996) (59). Bread volume was determined by the AACC (2003) method (60), and the specific volume was calculated as the ratio of volume to weight.
Mass loss after baking was calculated as a percentage by measuring loaf dough mass before baking and after baking. The bread shape retention coefficient was calculated as the ratio of bread slice width to height (in mm). Crust and crumb color parameters were evaluated as described in Section 2.3.
Bread crumb hardness was determined as the energy required for sample deformation (CT3 Texture Analyzer, Brookfield, USA): bread slices of 2 cm thickness were compressed to 10% of their original height at a crosshead speed of 0.5 mm/s; the resulting peak energy of compression was reported as crumb hardness. Three replicates from two different sets of baking were analyzed and averaged.
The saccharide content in bread was determined according to the method described in Section 2.3.
Acrylamide content was analyzed according to the method of Zhang et al. (61) with some modifications as described in Supplementary File S1. The compound was determined on the basis of derivatization of the target analyte with bromination. Analysis was performed with a gas chromatograph–electron capture detector (GC–ECD), using the acrylamide analytical standard.
2.5. Statistical analysis
The results were expressed as the mean values (for baking dough and bread samples n = 6, and for bread sensory characteristics and overall acceptability n = 10 trained panelists) ± standard error (SE). In order to evaluate the effects of different amounts of psyllium husk gel on bread quality parameters, data were analyzed using a one-way analysis of variance (ANOVA) and Tukey’s-honest significant difference (Tukey-HSD) as post hoc tests (statistical program R 3.2.1) (62). In addition, Pearson correlations were calculated between various parameters (63). The results were recognized as statistically significant at a p level equal to or below 0.05 (p ≤ 0.05).
3. Results and discussion
3.1. Influence of psyllium husk gel on baking dough color coordinates and concentration of saccharides
The chromaticity parameters of the psyllium husk gel and dough samples are tabulated in Table 1. Regarding the L* coordinate [brightness or (−L*) darkness], the lowest L* value was obtained in psyllium husk gel samples, and comparing among dough sample groups, different trends were found: the lowest L* value (84.4 NBS) was found in baking dough prepared with 15% of psyllium husk gel. Control baking doughs and dough groups prepared with 10 and 25% of psyllium husk gel showed similar L* values (on average, 85.6 NBS), whereas the highest L* values (on average, 86.5 NBS) were reached in baking dough groups prepared with 5 and 20% of psyllium husk gel.
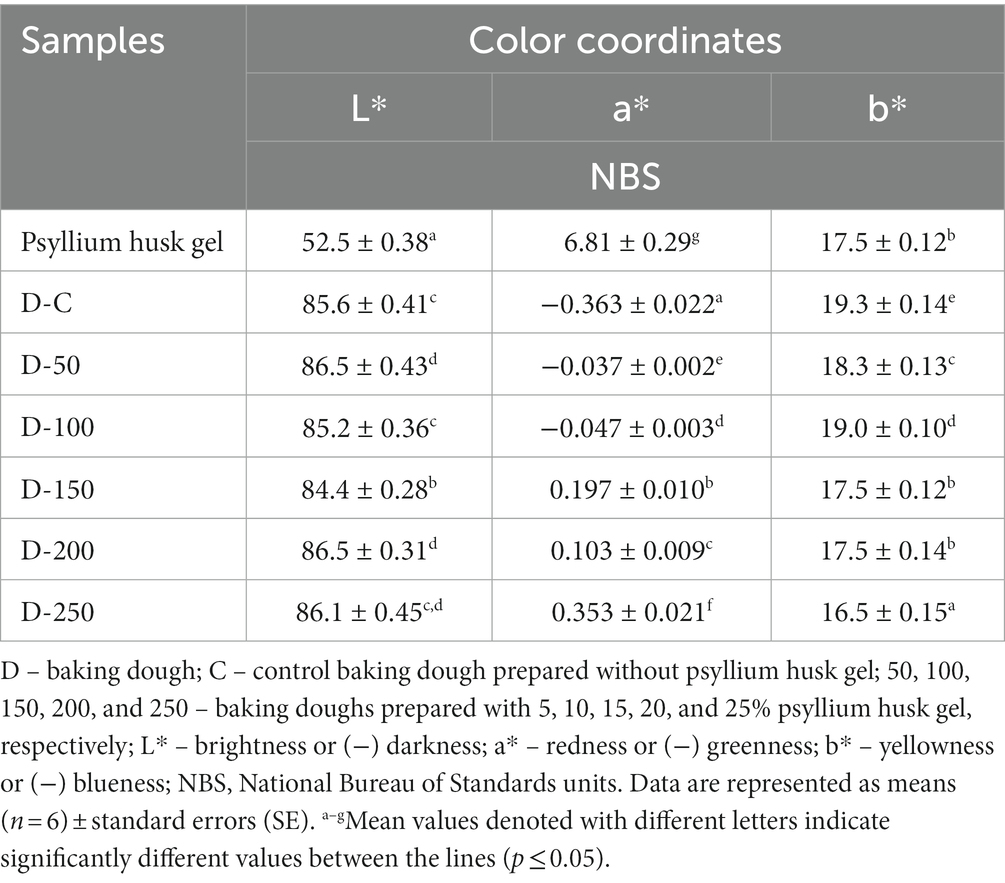
Table 1. Chromaticity parameters of psyllium husk gel and baking dough samples.
In comparison, a* coordinate [redness or (−a*) greenness] of baking dough samples and despite the highest redness value (6.81 NBS) found in the psyllium husk gel, three out of six dough sample groups showed higher expression of greenness and then redness (control doughs and sample groups prepared with 5 and 10% of psyllium husk gel). Samples prepared with 25% of psyllium husk gel showed the highest redness coordinates (0.353 NBS), and samples prepared with 15 and 20% of psyllium husk gel presented the lowest redness coordinates (on average, 1.79 and 3.43 times lower, respectively).
Concerning the b* coordinate [yellowness or (−b*) blueness], the highest values (19.3 NBS) were observed in the control samples, whereas the other samples showed values lower than 4.5 and 1.55% (samples prepared with 25 and 10% of psyllium husk gel, respectively). Finally, tests of between-subject effects showed that the amount of psyllium husk gel is a statistically significant factor on dough a* coordinate values (p < 0.001).
Information about the color coordinates of psyllium husk gel or its effect on dough chromaticity properties is scarce. However, it was reported that psyllium husk powder L*, a*, and b* coordinate values were 66.64, 4.01, and 12.36 NBS, respectively (64). In addition, the data about the influence of psyllium husk on noodle dough stated that L* and b* values decrease while a* value increases by increasing its content in the dough (40). Another study revealed that psyllium husk added to sponge cake led to an increase in the dough L* and a* values and a decrease in the b* value (65). Gómez et al. testified that fiber concentration is responsible for the change in a* and b* parameters in baking doughs (66). Differences in color coordinates of tested doughs likely occurred due to the natural pigments present in psyllium husk gel and different mechanisms of its action in the baking dough, including its influence on proteins and enzyme activities. However, further, deeper research is required to evaluate the exact influence of psyllium husk gel on the chromaticity parameters of baking dough.
The concentration of sugars (g/100 g) in baking dough samples is shown in Figure 2. Among all analyzed sugars (fructose, glucose, sucrose, and maltose) only maltose could be detected in the baking dough samples. The lowest content of maltose was found in samples prepared with 10% of psyllium husk gel (0.810 g/100 g). Control samples and samples prepared with 20% of psyllium husk gel showed a slightly higher content of maltose but it was not statistically significant (on average, 0.895 g/100 g). In samples prepared with 5, 15, and 25% of psyllium husk gel, maltose content was, on average, 22.9% higher than in samples prepared with 10% of psyllium husk gel (the latter had the lowest maltose content).
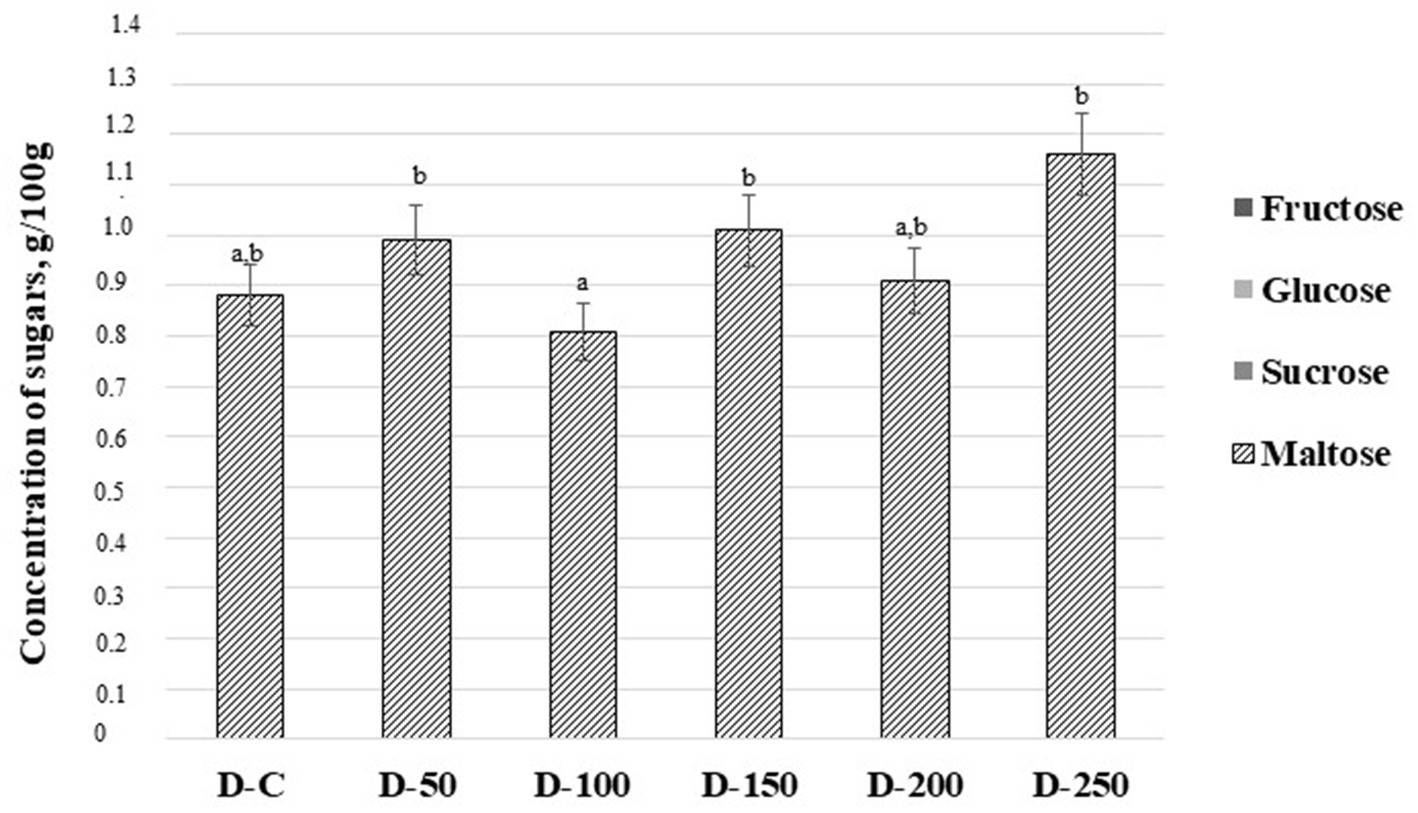
Figure 2. Concentration of sugars (g/100 g) in baking dough samples [D – baking dough; C – control baking dough prepared without psyllium husk gel; 50, 100, 150, 200, and 250 – baking doughs prepared with 5, 10, 15, 20, and 25% psyllium husk gel, respectively; Data are represented as means (n = 6) ± standard errors (SE); a–bMean values denoted with different letters indicate significantly different values between the columns (p ≤ 0.05)].
The predominant saccharides found in refined wheat flour are sucrose (2.16 ± 0.26 mg/g of flour), maltose (0.53 ± 0.09 mg/g of flour), fructose (0.91 ± 0.13 mg/g of flour), and glucose (0.54 ± 0.09 mg/g of flour) (67). Sucrose is rapidly hydrolyzed by yeast into glucose and fructose (68). In addition to glucose and fructose, maltose and raffinose can be found in flour, and polyols can also be synthesized by microorganisms present in doughs (69). During breadmaking, when yeasts are used for fermentation, the fermentable saccharides are converted into carbon dioxide and ethanol (70), with a preference for glucose consumption over fructose and maltose (69). This partly explains the low profile of detected sugars in tested doughs. Moreover, the fact that arabinoxylans in psyllium husk consist mostly of arabinose and xylose, as well as minor levels of rhamnose, galactose, and glucose, also contributes to the obtained results (37). Changes in maltose content between tested doughs could be explained by the fact that psyllium husk gel as dietary fiber/hydrocolloid could interact with dough constituents and entrap them or disrupt the internal relations (71). For example, the entrapment of starch could limit its availability for enzymes and sugar release. Nevertheless, there is currently no universal agreement on the mechanism of action of such hydrocolloids (72).
3.2. Influence of psyllium husk gel on the main parameters of bread
Scores of the bread’s overall acceptability and bread crumb images are presented in Figures 3, 4, respectively. In comparison with control bread, sample groups prepared with psyllium husk gel showed higher overall acceptability (Figure 3), chiefly, the overall acceptability of samples with 5% of psyllium husk gel was, on average, 17.7% higher, the overall acceptability of samples with 10, 15, and 20% of psyllium husk gel was, on average, 45.6% higher, and the overall acceptability of samples with 25% of psyllium husk gel was, on average, 21.0% higher than the control.
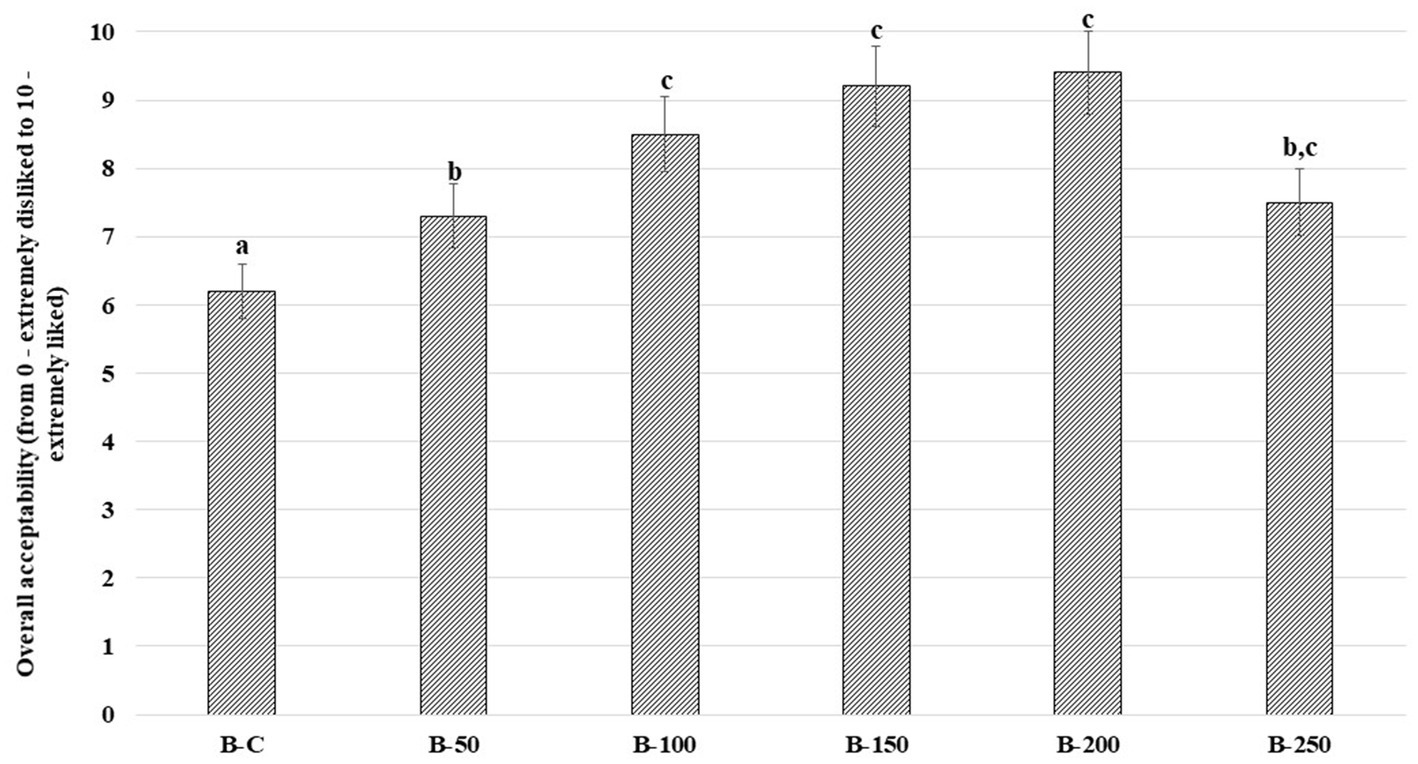
Figure 3. Overall acceptability (OA) of wheat bread (WB) [B – bread; C – control bread prepared without psyllium husk gel; 50, 100, 150, 200, and 250 – bread samples prepared with 5, 10, 15, 20, and 25% psyllium husk gel, respectively; Data are represented as means (n = 10) ± standard errors (SE); a–cMean values denoted with different letters indicate significantly different values between the columns (p ≤ 0.05)].
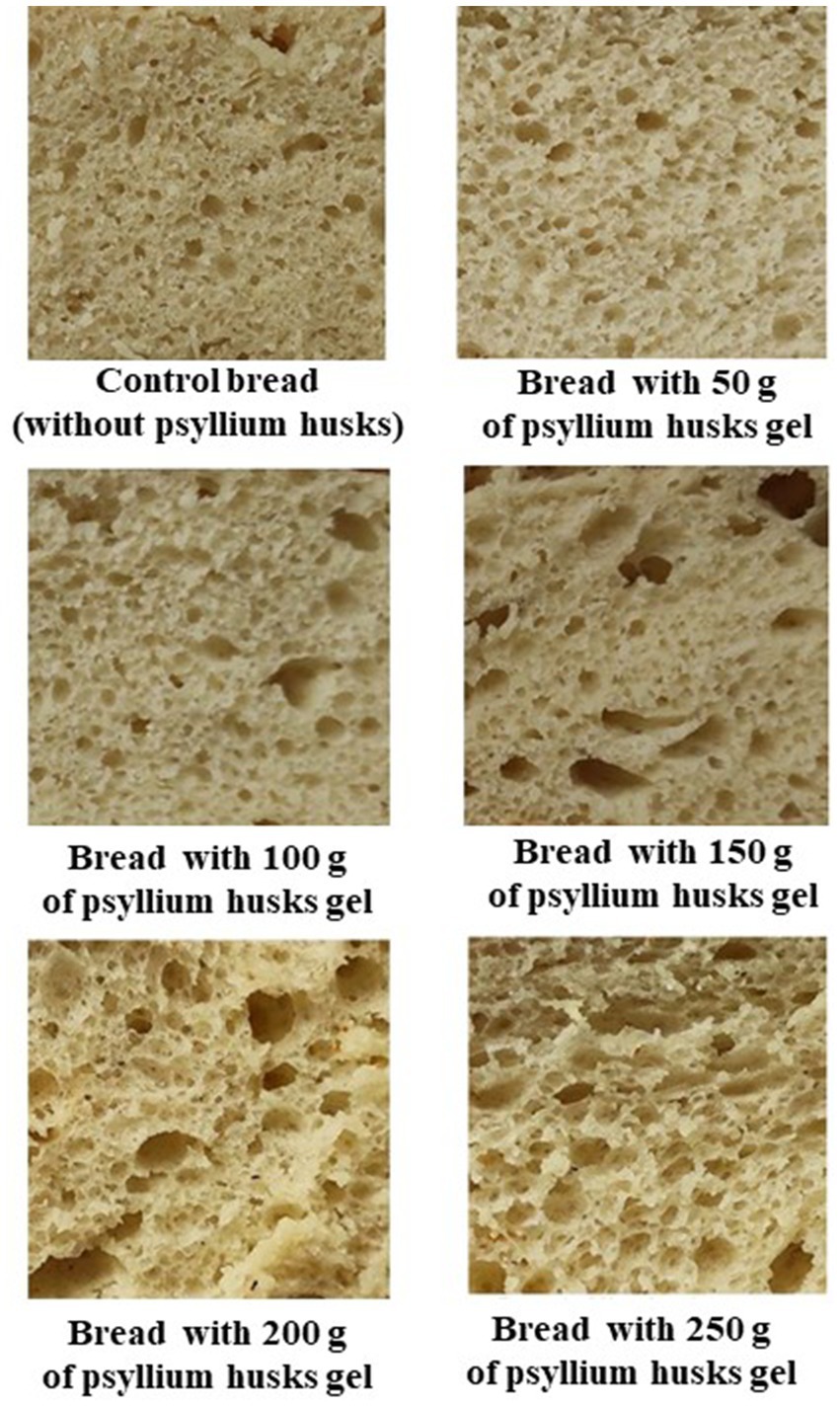
Figure 4. Images of wheat bread crumb samples.
The higher acceptability of tested bread with psyllium husk gel could be related to the softer texture and increased porosity of bread crumbs (73). Similarly to our results, it was conveyed that the incorporation of psyllium (at 2 or 5%) improved the taste of whole meal breads by increasing their overall acceptability (1). Furthermore, bread with a greater level of moisture has more juiciness, which consumers prefer, and that could also be the reason for the higher acceptability scores of tested breads in our study (57). Moriarty et al. suggested that highlighting the advantages of psyllium husk incorporation in the main bread recipes (e.g., fewer calories, higher amount of fiber, etc.), could be a lever to increase the acceptability of bread with psyllium (74). Lastly, psyllium husk gel, as an ingredient for refined wheat flour bread preparation, can increase the functional value and overall acceptability of such types of bread.
Observing the bread crumb images (Figure 4), a clear visual trend was perceived. In particular, by increasing psyllium husk gel amounts to the main bread recipe, the number of large pores in bread crumb increased. In relation to the retention coefficient of bread, samples prepared with 15 and 25% of psyllium husk gel showed lower shape retention coefficient values than the control bread and bread samples prepared with 5, 10, and 20% of psyllium husk gel (on average, 18.9% lower) (Table 2). Taking into consideration that the bread shape retention coefficient is calculated as the ratio between bread slice width and height, this means that the lower the shape retention coefficient values, the higher the height of the bread loaf. In addition, by increasing psyllium husk gel amounts to the main bread formula, the specific volume of bread increased. In comparison with control samples, bread prepared with 5 and 10% of psyllium husk gel showed, on average, 5.65% higher specific volume, bread with 15 and 20% of psyllium husk gel showed, on average, 12.3% higher specific volume, and bread with 25% of psyllium husk gel showed, on average, 16.6% higher specific volume. Similar tendencies were established for the porosity of bread samples, i.e., by increasing psyllium husk gel amounts in the main bread formula, the porosity of bread samples increased. Nevertheless, the correlation (r) between bread-specific volume and porosity was not statistically significant. However, bread porosity showed a moderate positive correlation with bread mass loss after baking (r = 0.567, p = 0.14).
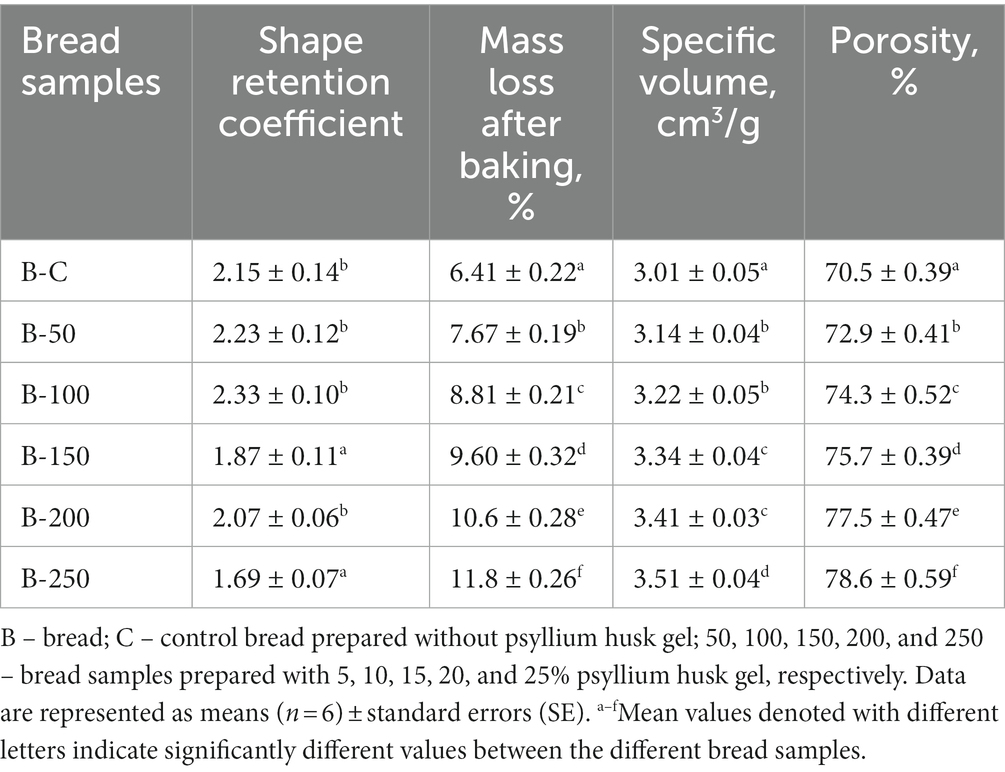
Table 2. Bread quality parameters: shape retention coefficient, mass loss (ML) after baking, specific volume (v), and porosity.
Reported data on the effect of psyllium husk on bread quality features are inconsistent. Our results are in agreement with Yassin et al., who observed that the addition of psyllium husk significantly increased bread loaf volume in comparison with control samples (75). Conversely, the results from Man et al. showed opposite tendencies, i.e., by increasing psyllium husk amounts, loaf-specific volume decreased, with these changes being explained by the dilution of the gluten network caused by psyllium fiber (76). The research from Mironeasa and Codina also showed that the specific volume of bread decreased as the concentration of psyllium increased (55). It should be mentioned that the difference in the reported data could be due to multiple factors, e.g., different processing steps, different ingredient characteristics, etc. In our study, the higher specific volume and porosity after baking bread with psyllium husk gel could be explained by the presence of water-soluble dietary fibers, which contribute to an additional polymeric network formation within the dough and, thus, led to a stronger and more extensible viscoelastic structure that improved the gas retention capacity of dough and increased loaf specific volume (75). Moreover, a greater water binding capacity in bread dough can reinforce the gluten network, possibly by giving a better plasticizing impact and minimizing regions of extremely compact gluten-gluten intermolecular relations (77). In terms of crumb structure, it was reported that higher concentrations of psyllium husk contribute to the lessened uniformity of the crumb structures with a tendency toward larger air cells (75), and these changes in crumb structures could contribute to the different mechanical and sensory characteristics of bread (78).
Changes in bread texture hardness during bread storage (12, 24, 48, and 72 h) are shown in Figure 5. Analyzing the bread texture hardness after 12 h of storage, it was unveiled that by increasing the psyllium husk gel amount to the main bread formula, the hardness was reduced. Moreover, in comparison with control samples, bread prepared with 5% of psyllium husk gel presented, on average, 20.0% lower crumb hardness, bread prepared with 10% of psyllium husk gel, showed, on average, 40.0% lower crumb hardness, and bread prepared with 15, 20, and 25% of psyllium husk gel disclosed, on average, 60.0% lower crumb hardness. After 24 h of storage, control samples showed hardness values similar to those obtained after 12 h of storage (0.500 mJ). However, bread prepared with 5 and 25% of psyllium husk gel showed increased hardness – on average, by 25 and 50%, respectively – when comparing samples with 12 and 24 h of storage.
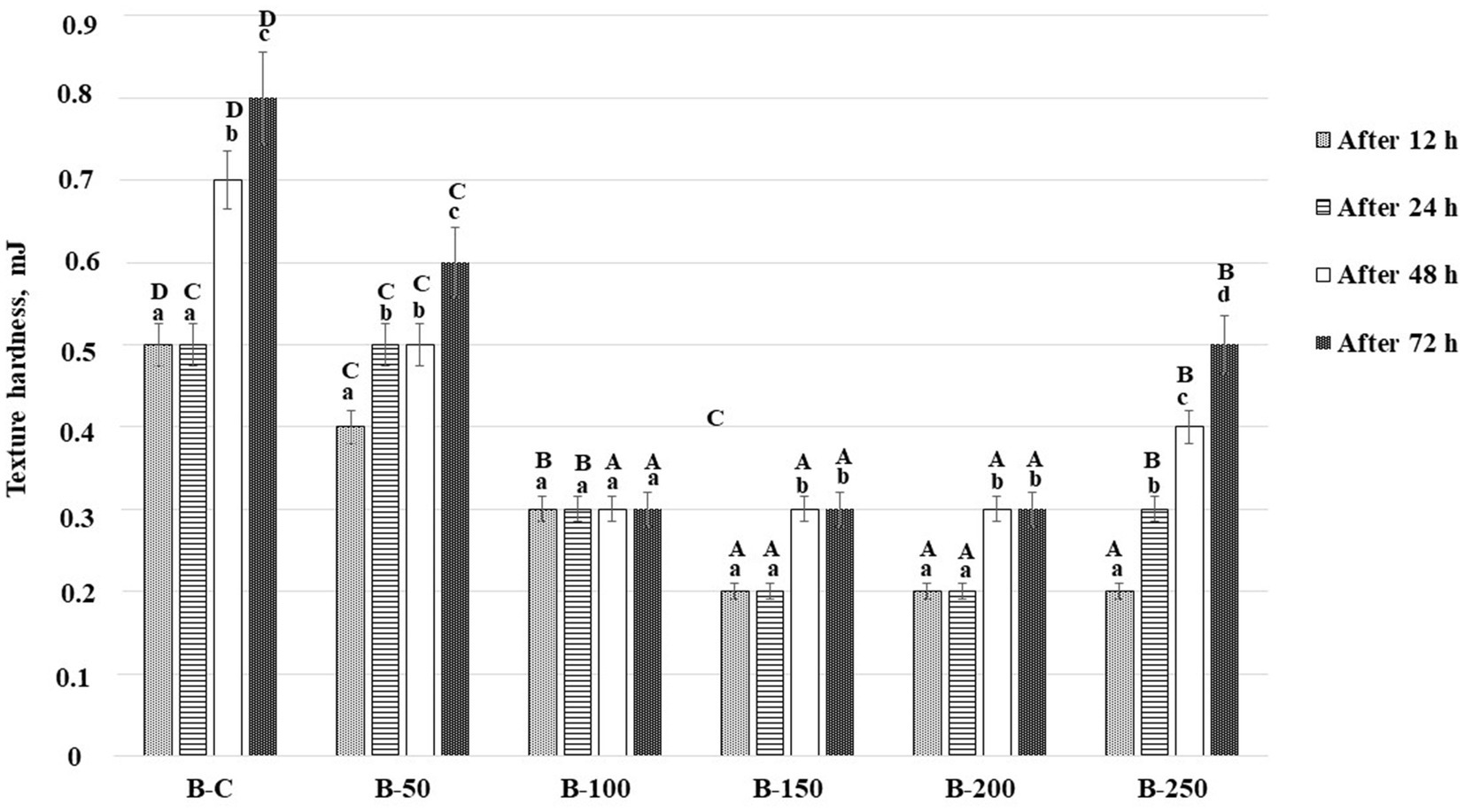
Figure 5. Changes in bread texture hardness (mJ) during storage [B – bread; C – control bread prepared without psyllium husk gel; 50, 100, 150, 200, and 250 – bread samples prepared with 5, 10, 15, 20, and 25% psyllium husk gel, respectively; Data are represented as means (n = 6) ± standard errors (SE); a–dMean values denoted with different letters indicate significantly different values between the same group of samples after different storage time period; A–DMean values denoted with different letters indicate significantly different values between the different bread samples after the same storage time period; (p ≤ 0.05)].
In comparison with the hardness of the same group of samples after 12 h, it was observed that after 48 h of storage, the hardness of the control bread increased, on average, by 1.4 times, the hardness of bread with 5% of psyllium husk gel increased, on average, by 1.25 times; that of bread with 10% of psyllium husk gel, remained similar, that of bread with 15 and 20% of psyllium husk gel increased, on average, by 1.5 times, and the hardness of bread with 25% of psyllium husk gel increased, on average, by 2.0 times. It is important to underline that despite the higher changes observed in bread prepared with 15, 20, and 25% of psyllium husk, their texture hardness after 48 h of storage was lower, on average, by 57.1% (bread with 15 and 20% of psyllium husk gel) and 42.9% (bread with 25% of psyllium husk gel), in comparison with the control bread samples. After 72 h of storage, similar tendencies were seen. In all cases, the hardest structure was obtained in the control samples and the lowest hardness was found on bread prepared with 10, 15, and 20% of psyllium husk gel (on average, 0.300 mJ).
Usually, during storage, the bread hardness increases because of the starch retrogradation process. However, the staling process is slower in bread prepared with high water-binding capacity components (75). In breadmaking, psyllium husk is used as a technological improver, particularly to slow bread staling by changing and delaying the starch retrogradation process in wheat bread (50, 79). In our study, the softer bread texture during storage could be explained by PHG’s ability to retain moisture and the higher specific volume of bread (75, 80). Moreover, the anti-staling effect of PHG could be explained by its fiber’s hydrogen bonding to starch amylopectin, which inhibits the formation of crystalline structures (81). It was reported that psyllium husk improves the quality of gluten-free bread, including its quality during storage (82). Abdullah et al. found out that bread enriched with 5% of psyllium husk showed a softer texture, in comparison with controls (57). However, another study revealed that these changes are related to the bread formulation and the addition of 5% of psyllium husk to white wheat flour significantly softened the bun texture, whereas, the addition of the same quantity of psyllium husk to whole wheat flour resulted in a much harder texture of the bun (57). Yassin et al. reported that the non-starch polysaccharides lower bread hardness (75). Our results are in agreement with the abovementioned findings. Moreover, a strong positive correlation between the bread texture hardness (after 12 h of storage) and the porosity of the samples (r = 0.664, p = 0.003) was found in our study. Usually, higher porosity leads to a faster bread staling process due to faster water migration from the bread crumb. However, in this study, the obtained results showed the opposite, and it can be stated that hydrocolloids, in addition to the increased bread porosity, have a lowering effect on water migration in bread crumb and lead to a softer bread during storage.
3.3. Concentration of acrylamide in bread and its relation with bread crust and crumb chromaticity characteristics and content of sugars
Acrylamide concentration in bread samples is given in Figure 6. It is apparent in all cases that a lower content of acrylamide was obtained in bread prepared with psyllium husk gel. Samples prepared with 5, 10, 15, 20, and 25% of psyllium husk showed, respectively, on average, 1.53, 2.34, 3.80, 2.69, and 3.62 times lower acrylamide concentration than the control bread. A strong positive correlation was found between acrylamide content and the porosity of the bread (r = 0.672, p = 0.002). Yet, a moderate positive correlation was established between acrylamide content in bread and maltose concentration in dough samples (r = 0.567, p = 0.014) (Table 3).
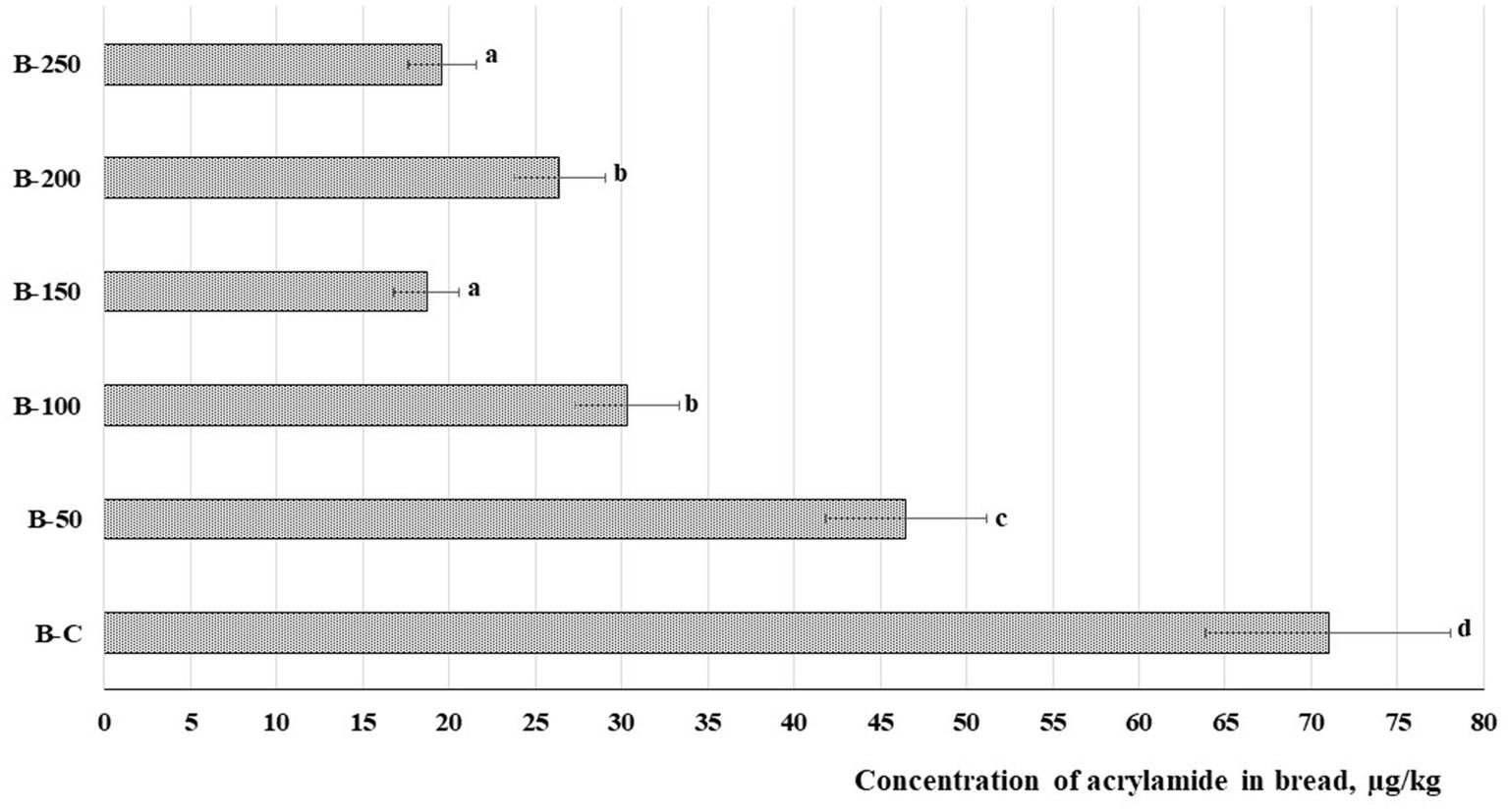
Figure 6. Concentration of acrylamide (μg/kg) in bread [B – bread; C – control bread prepared without psyllium husk gel; 50, 100, 150, 200, and 250 – bread samples prepared with 5, 10, 15, 20, and 25% psyllium husk gel, respectively; Data are represented as means (n = 6) ± standard errors (SE). a–dMean values denoted with different letters indicate significantly different values between the different bread samples (p ≤ 0.05)].
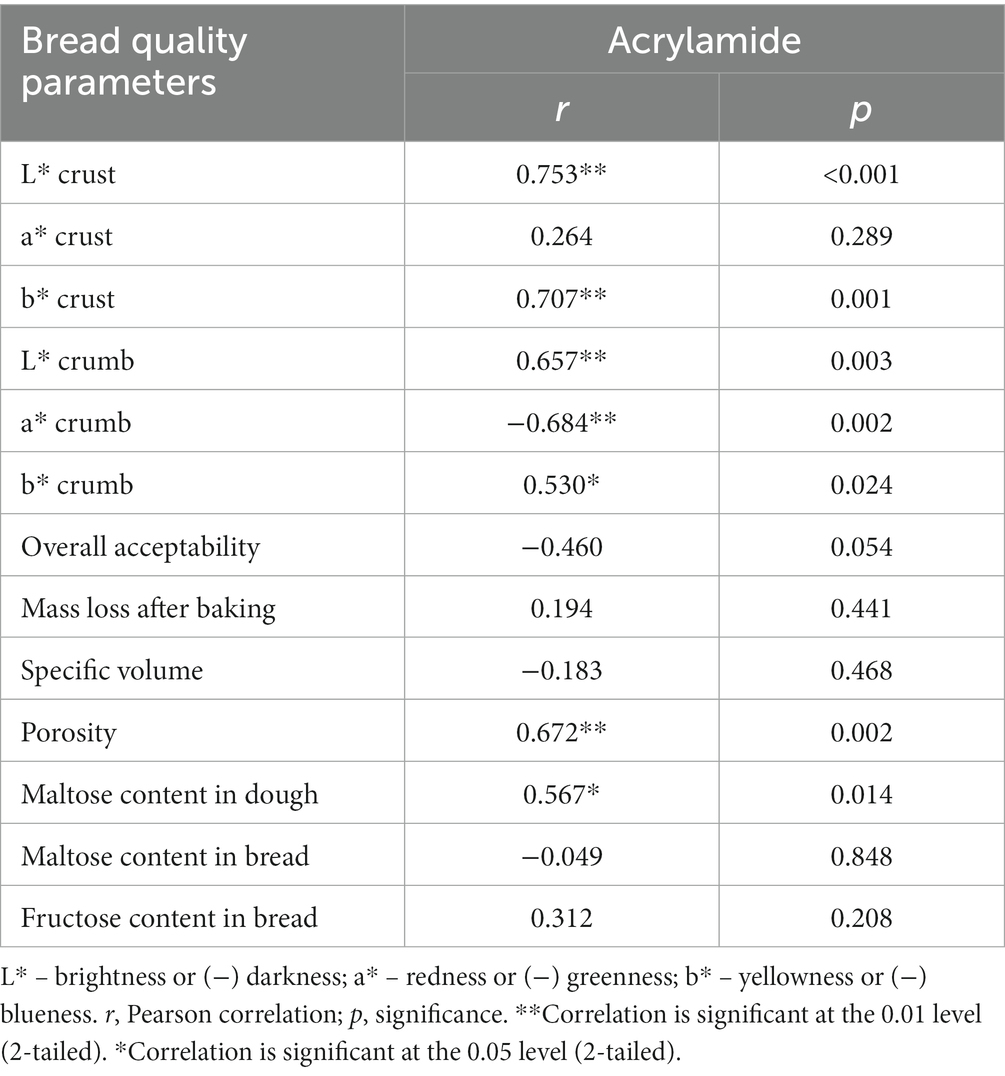
Table 3. Pearson correlations (r) between the content of acrylamide in bread and bread crumb and crust color coordinates, overall acceptability (OA), mass loss (ML) after baking, specific volume (v), porosity, and content of sugars in bread, and their significance (p).
The chromaticity parameters of bread crust and crumb are summarized in Table 4. Observing the L* values of bread crust, samples prepared with 15 and 25% of psyllium husk gel showed, respectively, on average, 3.50 and 12.9% lower L* coordinates than the control bread. In addition, bread prepared with 5, 10, and 20% of psyllium husk showed, respectively, on average, 20.3, 6.43, and 4.42% higher L* values than the control samples. A strong positive correlation was found between acrylamide content in samples and bread crust L* coordinate values (r = 0.753, p < 0.001) (Table 3).
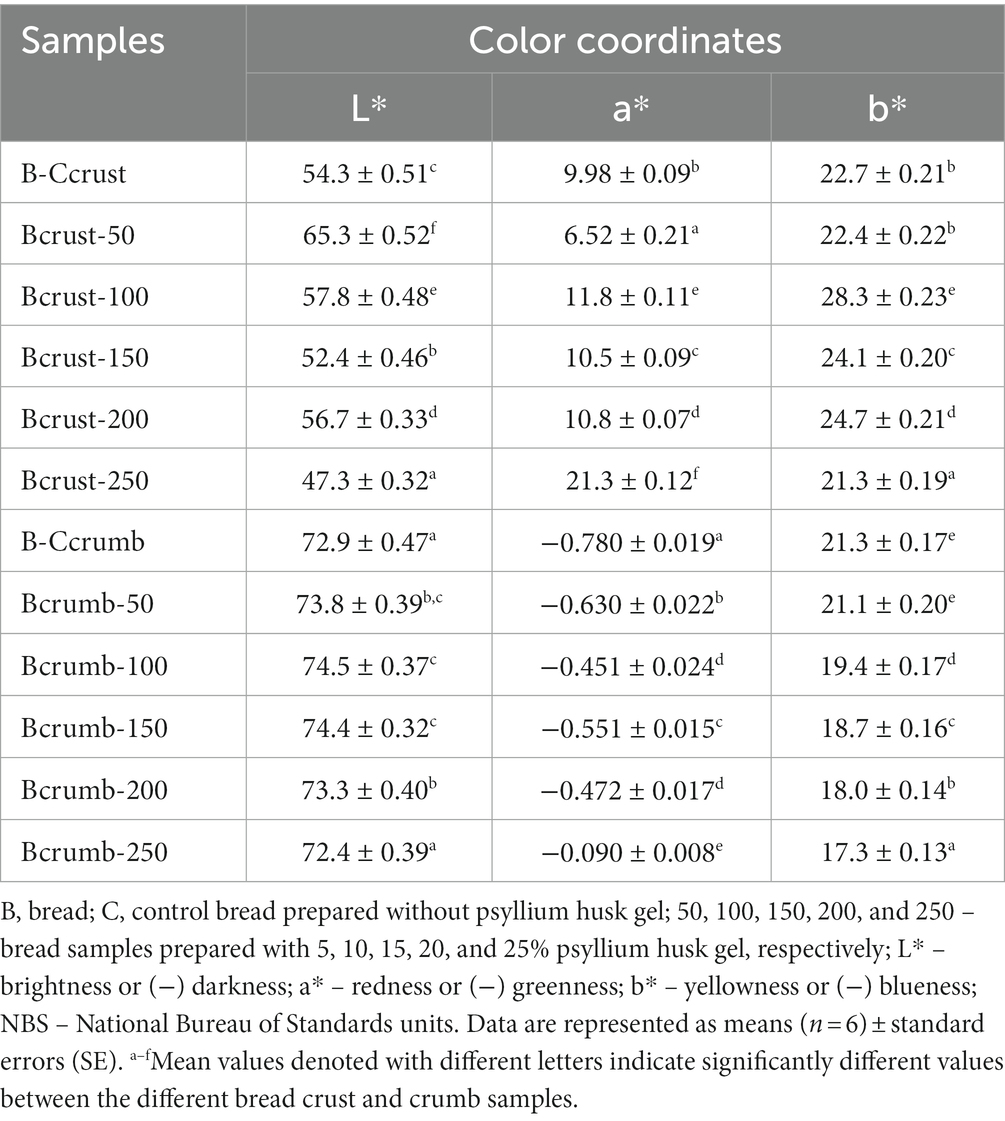
Table 4. Chromaticity (L*, a*, and b*) parameters in bread crust and crumb.
Regarding bread crust a* values, samples prepared with 5% of psyllium husk gel showed, on average, 34.7% lower a* coordinates than the control breads. However, other bread groups prepared with psyllium husk gel, presented significantly higher a* values than the control bread crust samples. The lowest crust b* coordinates (21.3 NBS) were found in bread prepared with 25% of psyllium husk gel. The b* coordinates were, on average, 22.6 NBS in the control bread and samples prepared with 5% of psyllium husk gel. Other bread crust samples (viz., with 10, 15, and 20% of psyllium husk gel) showed, respectively, on average, 25.2, 6.64, and 9.29% higher b* values than the control bread and bread prepared with 5% of psyllium husk gel. A strong positive correlation was attained between acrylamide content and bread and bread crust b* values (r = 0.707, p = 0.001) (Table 3). In comparison, bread crumb color coordinates and the lowest L* values were achieved in control samples and samples prepared with 25% of psyllium husk gel (on, average, 72.7 NBS), whereas the lowest a* coordinates were found in control samples (−0.780 NBS), and the lowest b* values were established in bread prepared with 25% of psyllium husk gel (17.3 NBS). A strong positive correlation was obtained between acrylamide content in bread samples and bread crumb L* values (r = 0.657, p = 0.003), a strong negative correlation was established between acrylamide content in bread samples and bread crumb a* values (r = −0.684, p = 0.002), and a moderate positive correlation was obtained between acrylamide content in bread samples and bread crumb b* coordinates (r = 0.530, p = 0.024) (Table 3).
Surdyk et al. reported a strong correlation between acrylamide content in bread and crust color (83). Likewise, Dessev et al. indicated a strong positive correlation between total color difference and acrylamide content in bread (84).
The concentration of sugars (g/100 g) in bread samples is depicted in Figure 7. From all analyzed sugars (fructose, glucose, sucrose, and maltose) only maltose and fructose were found in bread samples. Maltose content in bread samples varied and the highest concentration was established in bread prepared with 25% of psyllium husk gel (1.61 g/100 g). The highest concentration of fructose was quantified in bread prepared with 5% of psyllium husk gel (0.541 g/100 g). It is worth noting that fructose content was slightly lower in samples prepared with 10% of psyllium husk gel but it was not statistically different. Moreover, significant correlations between acrylamide and the content in bread samples of analyzed sugars were not established (Table 3).
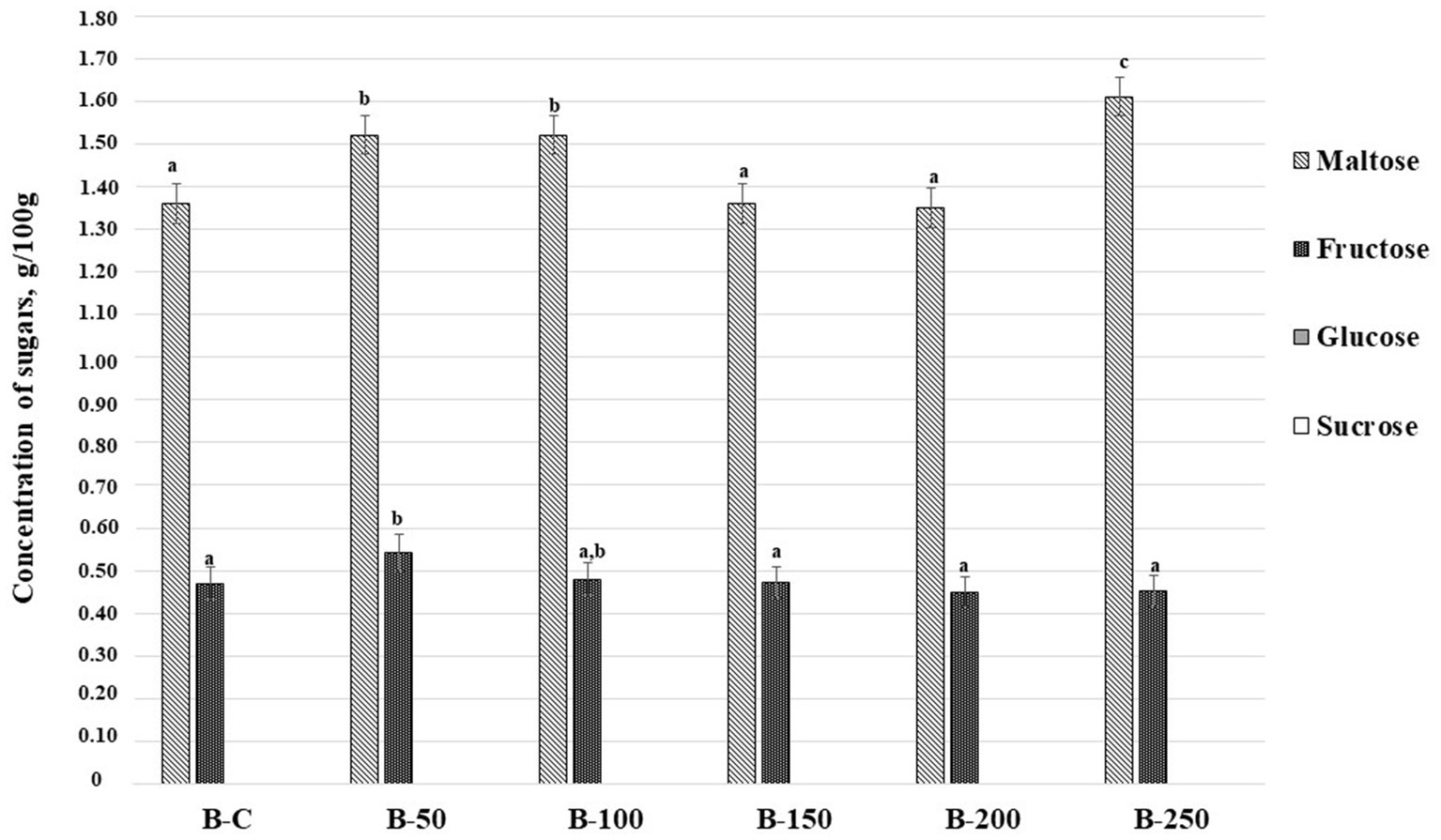
Figure 7. Concentration of sugars (g/100 g) in bread samples [B – bread; C – control bread prepared without psyllium husk gel; 50, 100, 150, 200, and 250 – bread samples prepared with 5, 10, 15, 20, and 25% psyllium husk gel, respectively; Data are represented as means (n = 6) ± standard errors; a–cMean values denoted with different letters indicate significantly different values between the different sugars (p ≤ 0.05)].
The reduction or dilution of acrylamide precursors in baking dough is considered a prospective strategy to decrease acrylamide formation in the final bread (29). Maillard reaction, as a non-enzymatic browning, is involved with desirable properties in cereal-based products, chiefly color and aroma (85). During the first stages of acrylamide formation, the interaction between carbonyl and amino groups results in Schiff bases (29). The latter rearrange to Amadori or Heyns products (86). However, the Schiff bases may be decarboxylated or hydrolyzed and form azomethine ylide as well as 3-aminopropionamide. Additionally, acrylamide may be formed via deamination of 3-aminopropionamide or directly by azomethine ylide (87, 88). Constituents of the flour play major roles in acrylamide formation, and acrylamide is formed in higher concentrations in bread manufactured with high extraction rate flours (89). For this reason, improving the functional value of bread by incorporating the outer layer of cereal dietary fiber becomes very challenging and, to overcome related difficulties, new ingredients such as psyllium husks can be very promising. Additionally, to reduce acrylamide concentration in bread, technological solutions such as reducing baking temperatures and, simultaneously, prolonging the baking process may be suggested, because high temperatures and low water activities are the major factors favoring acrylamide formation (90). However, during baking, crumb and crust temperatures of bread are different; as a reference, at the end of the process, the crust, on average, reaches 200°C, whereas the crumb reaches, on average, 98°C. For this reason, the crust shows a lower water activity than the crumb and, consequently, acrylamide concentration in the crumb is much lower than in the crust (91). Moreover, baking time and temperature are related to the rate of water loss from the surface of the bread (92), as well as higher bread porosity being able to lead to more water migration. Nevertheless, the brown color and crispy texture of the crust are important characteristics in the overall acceptability of bread (90) and, for this reason, in the current study, acrylamide analysis of bread loaf was determined to better meet a strategy for bread consumption.
Despite the temperature of the crumb being much lower than in the crust, Maillard and caramelization reactions during baking are the main factors for the formation of colors in bread crumb, although variations in color may be related to different contents of distinct pigments (93). Additionally, a compacted structure of crumb leads to lower lightness with a simultaneous increase in redness and yellowness (94). Our current research showed that there is a strong positive correlation between acrylamide content in samples and the porosity of the bread (Table 3). Despite that there are many recommendations for acrylamide reduction in bread, by reducing temperature and prolonging the duration of the baking process (30, 83, 84, 95–102), it should be highlighted that these technological solutions can lead to lower acceptability of the final baking products because of the low intensity of the crust color. The latter characteristic depends strongly on the temperature: the higher the surface temperature, the higher the moisture loss of the surface layers, and the more effective the Maillard reaction, thus, resulting in the darker color of the bread crust (103). Evaporation of water is related to the dough temperature reached during baking, and when any part of the baking dough reaches a temperature significantly above the boiling point, its water content becomes so low that evaporation is restricted and the balance between heat absorption and evaporation loss is altered. After this stage, the process of crust formation starts (84). However, our study showed that psyllium husk gel increases the softness of the bread and it is involved in the reduction of the moisture migration process of bread samples with higher porosity (bread porosity showed moderate positive correlation with bread mass loss after baking). Finally, in accordance with the lower acrylamide concentration obtained in bread prepared with psyllium husk gel, it can be stated that the psyllium husk gel hydrocolloids reduce water migration from bread during baking and, at the same time, reduce acrylamide formation in the end products.
4. Conclusion
This research paper provides an application of different quantities of psyllium husk gel to wheat bread production and evaluates its effect on bread quality and the formation of acrylamide, which is a neurotoxic and carcinogenic compound. Results revealed that psyllium husk gel improved the overall acceptability of the wheat bread from refined flour, and by increasing the amount of psyllium husk gel in the main bread recipe, specific volume and porosity increased. Bread containing psyllium husk gel had a softer crumb in all cases. Moreover, positive correlations were identified between porosity and mass loss after baking or texture hardness. Although the higher porosity of the bread usually leads to a faster staling process due to the faster water migration from the crumb, the results of our study were the opposite. Psyllium husk gel hydrocolloids showed, in addition to an increased bread porosity, a reducing effect on water migration from crumb and a softer texture during bread storage. Acrylamide formation was significantly diminished by the addition of psyllium husk gel to wheat bread due to its ability to reduce water migration during baking. The outcome of this study opens avenues for the application of psyllium husk gel in obtaining safer and higher-quality wheat bread.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
EB: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing. GK: Formal analysis, Investigation, Writing – original draft. VS: Data curation, Investigation, Methodology, Validation, Writing – original draft. DK: Formal analysis, Investigation, Methodology, Writing – original draft. EZ: Formal analysis, Software, Writing – original draft. DC: Formal analysis, Writing – original draft. EK: Data curation, Investigation, Writing – original draft. FÖ: Investigation, Writing – review & editing. JR: Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This work is based upon the work from COST Action CA21149 ACRYRED – Reducing acrylamide exposure of consumers by a cereals supply-chain approach targeting asparagine. COST is a funding agency for research and innovation networks. In addition, this work is based upon the work from COST Action 18101 SOURDOMICS – Sourdough biotechnology network toward novel, healthier and sustainable food and bioprocesses (https://sourdomics.com/; https://www.cost.eu/actions/CA18101/, accessed on 23 August 2023), where the author EB is the Vice-Chair and leader of the working group 6 “Project design and development innovative prototypes of products and small-scale processing technologies,” the author FÖ is the leader of the working group 8 “Food safety, health-promoting, sensorial perception and consumers’ behavior,” and the author JR is the Chair and Grant Holder Scientific Representative and is supported by COST (European Cooperation in Science and Technology) (https://www.cost.eu/, accessed on 23 August 2023). COST is a funding agency for research and innovation networks.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1277980/full#supplementary-material
References
2. Gómez, M, Gutkoski, LC, and Bravo-Núñez, Á. Understanding whole-wheat flour and its effect in breads: a review. Compr Rev Food Sci Food Saf. (2020) 19:3241–65. doi: 10.1111/1541-4337.12625
3. Barros, JHT, Telis, VRN, Taboga, S, and Franco, CML. Resistant starch: effect on rheology, quality, and staling rate of white wheat bread. J Food Sci Technol. (2018) 55:4578–88. doi: 10.1007/s13197-018-3393-6
4. Li, H, Dai, F, Zhang, L, and Li, Z. Characterization of scalded dough and its impact on the growth of mixed yeasts originating from Jiaozi. Food Biosci. (2022) 49:101920. doi: 10.1016/j.fbio.2022.101920
5. Klupsaite, D, Starkute, V, Zokaityte, E, Cernauskas, D, Mockus, E, Kentra, E, et al. The contribution of scalded and scalded-fermented Rye Wholemeal flour to quality parameters and acrylamide formation in semi-wheat-Rye bread. Foods. (2023) 12:937. doi: 10.3390/foods12050937
6. Bartkiene, E, Bartkevics, V, Krungleviciute, V, Pugajeva, I, Zadeike, D, Juodeikiene, G, et al. The influence of scalded flour, fermentation, and plants belonging to Lamiaceae Family on the wheat bread quality and acrylamide content. J Food Sci. (2018) 83:1560–8. doi: 10.1111/1750-3841.14176
7. Esteller, MS, and Lannes, SC. Production and characterization of sponge-dough bread using scalded Rye. J Texture Stud. (2008) 39:56–67. doi: 10.1111/j.1745-4603.2007.00130.x
9. Zhou, M, and Li, Z. Characteristics of scalded dough fermented by co-cultures of Saccharomyces cerevisiae Y10, Wickerhamomyces anomalus Y13 and Torulaspora delbrueckii Y22. Int J Food Sci Technol. (2021) 56:5923–30. doi: 10.1111/ijfs.15232
10. Sarand, I, Traksmaa, A, Klava, D, Kunkulberga, D, Straumite, E, Galoburda, R, et al. Traditional breads from the Baltic countries (Estonia, Latvia, Lithuania) In: M Garcia-Vaquero, K Pastor, GE Orhun, A Mcelhatton, and R JMF, editors. Traditional European breads: An illustrative compendium of ancestral knowledge and cultural heritage [internet]. Cham: Springer International Publishing (2023). 41–59.
12. Bartkiene, E, Rimsa, A, Zokaityte, E, Starkute, V, Mockus, E, Cernauskas, D, et al. Changes in the physicochemical properties of chia (Salvia hispanica L.) seeds during solid-state and submerged fermentation and their influence on wheat bread quality and sensory profile. Foods. (2023) 12:2093. doi: 10.3390/foods12112093
13. Bartkiene, E, Zokaityte, E, Kentra, E, Starkute, V, Klupsaite, D, Mockus, E, et al. Characterisation of lacto-fermented cricket (Acheta domesticus) flour and its influence on the quality parameters and acrylamide formation in wheat biscuits. Fermentation. (2023) 9:153. doi: 10.3390/fermentation9020153
14. Bartkiene, E, Zokaityte, E, Starkute, V, Zokaityte, G, Kaminskaite, A, Mockus, E, et al. Crickets (Acheta domesticus) as wheat bread ingredient: influence on bread quality and safety characteristics. Foods. (2023) 12:325. doi: 10.3390/foods12020325
15. Klupsaite, D, Kaminskaite, A, Rimsa, A, Gerybaite, A, Stankaityte, A, Sileikaite, A, et al. The contribution of new breed purple wheat (8526-2 and 8529-1) varieties Wholemeal flour and sourdough to quality parameters and acrylamide formation in wheat bread. Fermentation. (2022) 8:724. doi: 10.3390/fermentation8120724
16. Bartkiene, E, Jakobsone, I, Pugajeva, I, Bartkevics, V, Zadeike, D, and Juodeikiene, G. Reducing of acrylamide formation in wheat biscuits supplemented with flaxseed and lupine. LWT-Food Sci Technol. (2016) 65:275–82. doi: 10.1016/j.lwt.2015.08.002
17. Bartkiene, E, Bartkevics, V, Pugajeva, I, Krungleviciute, V, Mayrhofer, S, and Domig, K. Parameters of rye, wheat, barley, and oat sourdoughs fermented with Lactobacillus plantarum LUHS135 that influence the quality of mixed rye–wheat bread, including acrylamide formation. Int J Food Sci Technol. (2017) 52:1473–82. doi: 10.1111/ijfs.13412
18. Bartkiene, E, Bartkevics, V, Pugajeva, I, Krungleviciute, V, Mayrhofer, S, and Domig, K. The contribution of P. acidilactici, L. plantarum, and L. curvatus starters and L-(+)-lactic acid to the acrylamide content and quality parameters of mixed rye – wheat bread. LWT. (2017) 80:43–50. doi: 10.1016/j.lwt.2017.02.005
19. Bartkiene, E, Bartkevics, V, Krungleviciute, V, Pugajeva, I, Zadeike, D, and Juodeikiene, G. Lactic acid Bacteria combinations for wheat sourdough preparation and their influence on wheat bread quality and acrylamide formation. J Food Sci. (2017) 82:2371–8. doi: 10.1111/1750-3841.13858
20. Juodeikiene, G, Zadeike, D, Vidziunaite, I, Bartkiene, E, Bartkevics, V, and Pugajeva, I. Effect of heating method on the microbial levels and acrylamide in corn grits and subsequent use as functional ingredient for bread making. Food Bioprod Process. (2018) 112:22–30. doi: 10.1016/j.fbp.2018.08.007
21. Bartkiene, E, Bartkevics, V, Lele, V, Pugajeva, I, Zavistanaviciute, P, Mickiene, R, et al. A concept of mould spoilage prevention and acrylamide reduction in wheat bread: application of lactobacilli in combination with a cranberry coating. Food Control. (2018) 91:284–93. doi: 10.1016/j.foodcont.2018.04.019
22. Bartkiene, E, Jakobsone, I, Juodeikiene, G, Vidmantiene, D, Pugajeva, I, and Bartkevics, V. Effect of fermented Helianthus tuberosus L. tubers on acrylamide formation and quality properties of wheat bread. LWT Food Sci Technol. (2013) 54:414–20. doi: 10.1016/j.lwt.2013.05.015
23. Bartkiene, E, Jakobsone, I, Juodeikiene, G, Vidmantiene, D, Pugajeva, I, and Bartkevics, V. Effect of lactic acid fermentation of lupine wholemeal on acrylamide content and quality characteristics of wheat-lupine bread. Int J Food Sci Nutr. (2013) 64:890–6. doi: 10.3109/09637486.2013.805185
24. Bartkiene, E, Jakobsone, I, Juodeikiene, G, Vidmantiene, D, Pugajeva, I, and Bartkevics, V. Study on the reduction of acrylamide in mixed rye bread by fermentation with bacteriocin-like inhibitory substances producing lactic acid bacteria in combination with aspergillus Niger glucoamylase. Food Control. (2013) 30:35–40. doi: 10.1016/j.foodcont.2012.07.012
25. Bartkiene, E, Jakobsone, I, Pugajeva, I, Bartkevics, V, Vidmantiene, D, and Juodeikiene, G. Influence of the addition of Helianthus tuberosus L. fermented with different lactobacilli on acrylamide content in biscuits. Int J Food Sci Technol. (2015) 50:431–9. doi: 10.1111/ijfs.12643
28. Basaran, B, Anlar, P, Yılmaz Oral, ZF, Polat, Z, and Kaban, G. Risk assessment of acrylamide and 5-hydroxymethyl-2-furfural (5-HMF) exposure from bread consumption: Turkey. J Food Compos Anal. (2022) 107:104409. doi: 10.1016/j.jfca.2022.104409
29. Mollakhalili-Meybodi, N, Khorshidian, N, Nematollahi, A, and Arab, M. Acrylamide in bread: a review on formation, health risk assessment, and determination by analytical techniques. Environ Sci Pollut Res. (2021) 28:15627–45. doi: 10.1007/s11356-021-12775-3
30. Lineback, DR, Coughlin, JR, and Stadler, RH. Acrylamide in foods: a review of the science and future considerations. Annu Rev Food Sci Technol. (2012) 3:15–35. doi: 10.1146/annurev-food-022811-101114
31. Mesias, M, Delgado-Andrade, C, and Morales, FJ. An updated view of acrylamide in cereal products. Curr Opin Food Sci. (2022) 46:100847. doi: 10.1016/j.cofs.2022.100847
33. Mesias, M, Nouali, A, Delgado-Andrade, C, and Morales, FJ. How far is the Spanish snack sector from meeting the acrylamide regulation 2017/2158? Foods. (2020) 9:247. doi: 10.3390/foods9020247
34. Gunduz, B, and Pelin, C. Formulation and processing strategies to reduce acrylamide in thermally processed cereal-based foods. Int J Environ Res Public Health. (2023) 20:6272. doi: 10.3390/ijerph20136272
35. Belorio, M, Marcondes, G, and Gómez, M. Influence of psyllium versus xanthan gum in starch properties. Food Hydrocoll. (2020) 105:105843. doi: 10.1016/j.foodhyd.2020.105843
36. Franco, EAN, Sanches-Silva, A, Ribeiro-Santos, R, and de Melo, NR. Psyllium (Plantago ovata Forsk): from evidence of health benefits to its food application. Trends Food Sci Technol. (2020) 96:166–75. doi: 10.1016/j.tifs.2019.12.006
37. Waleed, M, Saeed, F, Afzaal, M, Niaz, B, Raza, MA, Hussain, M, et al. Structural and nutritional properties of psyllium husk arabinoxylans with special reference to their antioxidant potential. Int J Food Prop. (2022) 25:2505–13. doi: 10.1080/10942912.2022.2143522
38. Nešić, A, Cabrera-Barjas, G, Dimitrijević-Branković, S, Davidović, S, Radovanović, N, and Delattre, C. Prospect of polysaccharide-based materials as advanced food packaging. Molecules. (2019) 25:135. doi: 10.3390/molecules25010135
39. Noguerol, AT, Marta Igual, M, and Pagán, MJ. Developing psyllium fibre gel-based foods: physicochemical, nutritional, optical and mechanical properties. Food Hydrocoll. (2022) 122:107108. doi: 10.1016/j.foodhyd.2021.107108
40. Bak, SL, Cha, SH, Park, SB, Jiang, S, Hyun, TK, and Jang, KI. Quality characteristics of noodles produced using steam-treated dough prepared with psyllium husk and soaked-and-dried soybean. J Food Process Preserv. (2023) 2023:e5351057. doi: 10.1155/2023/5351057
41. Guillon, F, and Champ, M. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res Int. (2000) 33:233–45.
42. Jane, M, McKay, J, and Pal, S. Effects of daily consumption of psyllium, oat bran and poly Glycople X on obesity-related disease risk factors: a critical review. Nutrition. (2019) 57:84–91. doi: 10.1016/j.nut.2018.05.036
43. Ren, Y, Yakubov, GE, Linter, BR, MacNaughtan, W, and Foster, TJ. Temperature fractionation, physicochemical and rheological analysis of psyllium seed husk heteroxylan. Food Hydrocoll. (2020) 104:105737. doi: 10.1016/j.foodhyd.2020.105737
44. Singh, B. Psyllium as therapeutic and drug delivery agent. Int J Pharm. (2007) 334:1–14. doi: 10.1016/j.ijpharm.2007.01.028
45. Yadav, N, Sharma, V, Kapila, S, Malik, RK, and Arora, S. Hypocholesterolaemic and prebiotic effect of partially hydrolysed psyllium husk supplemented yoghurt. J Funct Foods. (2016) 24:351–8. doi: 10.1016/j.jff.2016.04.028
46. Al-Hamadani, YA, Yusoff, MS, Umar, M, Bashir, MJ, and Adlan, MN. Application of psyllium husk as coagulant and coagulant aid in semi-aerobic landfill leachate treatment. J Hazard Mater. (2011) 190:582–7. doi: 10.1016/j.jhazmat.2011.03.087
47. Zhang, J, Wen, C, Zhang, H, and Duan, Y. Review of isolation, structural properties, chain conformation, and bioactivities of psyllium polysaccharides. Int J Biol Macromol. (2019) 139:409–20. doi: 10.1016/j.ijbiomac.2019.08.014
49. Zokaityte, E, Siriakovaite, K, Starkute, V, Zavistanaviciute, P, Lele, V, Mozuriene, E, et al. Characteristics of nutraceutical chewing candy formulations based on fermented Milk permeate, psyllium husk, and apple by-products. Foods. (2021) 10:777. doi: 10.3390/foods10040777
50. Filipčev, B, Pojić, M, Šimurina, O, Mišan, A, and Mandić, A. Psyllium as an improver in gluten-free breads: effect on volume, crumb texture, moisture binding and staling kinetics. LWT. (2021) 151:112156. doi: 10.1016/j.lwt.2021.112156
52. Mariotti, M, Lucisano, M, Pagani, MA, and Ng, PK. The role of corn starch, amaranth flour, pea isolate, and psyllium flour on the rheological properties and the ultrastructure of gluten-free doughs. Food Res Int. (2009) 42:963–75. doi: 10.1016/j.foodres.2009.04.017
53. Belorio, M, and Gómez, M. Psyllium: a useful functional ingredient in food systems. Crit Rev Food Sci Nutr. (2021) 62:527–38. doi: 10.1080/10408398.2020.1822276
54. Farbo, MG, Fadda, C, Marceddu, S, Conte, P, Del Caro, A, and Piga, A. Improving the quality of dough obtained with old durum wheat using hydrocolloids. Food Hydrocoll. (2020) 101:105467. doi: 10.1016/j.foodhyd.2019.105467
55. Mironeasa, S, and Codină, GG. Optimization of bread quality of wheat flour with psyllium addition by using response surface methodology. J Culin Sci Technol. (2023) 21:371–86. doi: 10.1080/15428052.2021.1948480
56. Pejcz, E, Spychaj, R, Wojciechowicz-Budzisz, A, and Gil, Z. The effect of Plantago seeds and husk on wheat dough and bread functional properties. Lwt. (2018) 96:371–7. doi: 10.1016/j.lwt.2018.05.060
57. Abdullah, MM, Aldughpassi, ADH, Sidhu, JS, Al-Foudari, MY, and Al-Othman, ARA. Effect of psyllium husk addition on the instrumental texture and consumer acceptability of high-fiber wheat pan bread and buns. Ann Agric Sci. (2021) 66:75–80. doi: 10.1016/j.aoas.2021.05.002
59. Bread and Bread Products. Porosity (LST 1442:1996). Vilnius: Lithuanian Standards Board (LST) (1996).
61. Zhang, Y, Dong, Y, Ren, Y, and Zhang, Y. Rapid determination of acrylamide contaminant in conventional fried foods by gas chromatography with electron capture detector. J Chromatogr A. (2006) 1116:209–16. doi: 10.1016/j.chroma.2006.03.042
62. Gillard, J. One-way analysis of variance (ANOVA) In: J Gillard, editor. A first course in statistical inference [internet]. Cham: Springer International Publishing (2020). 91–101.
63. Evans, JD. Straightforward statistics for the behavioral sciences, vol. xxii. Belmont, CA: Thomson Brooks/Cole Publishing Co (1996). 600 p.
64. Raymundo, A, Fradinho, P, and Nunes, MC. Effect of psyllium fibre content on the textural and rheological characteristics of biscuit and biscuit dough. Bioact Carbohydr Diet Fibre. (2014) 3:96–105. doi: 10.1016/j.bcdf.2014.03.001
65. Beikzadeh, S, Sh, P, Beikzadeh, M, AJA, M, and Homayouni-Rad, A. Effect of psyllium husk on physical, nutritional, sensory and staling properties of dietary prebiotic sponge cake. Czech J Food Sci. (2016) 34:534–40. doi: 10.17221/551/2015-CJFS
66. Gómez, M, Jiménez, S, Ruiz, E, and Oliete, B. Effect of extruded wheat bran on dough rheology and bread quality. LWT Food Sci Technol. (2011) 44:2231–7. doi: 10.1016/j.lwt.2011.06.006
67. Sahlström, S, Park, W, and Shelton, DR. Factors influencing yeast fermentation and the effect of LMW sugars and yeast fermentation on hearth bread quality. Cereal Chem. (2004) 81:328–35. doi: 10.1094/CCHEM.2004.81.3.328
68. Struyf, N, Laurent, J, Lefevere, B, Verspreet, J, Verstrepen, KJ, and Courtin, CM. Establishing the relative importance of damaged starch and fructan as sources of fermentable sugars in wheat flour and whole meal bread dough fermentations. Food Chem. (2017) 218:89–98. doi: 10.1016/j.foodchem.2016.09.004
69. Timmermans, E, Bautil, A, Brijs, K, Scheirlinck, I, Van der Meulen, R, and Courtin, CM. Sugar levels determine fermentation dynamics during yeast pastry making and its impact on dough and product characteristics. Foods. (2022) 11:1388. doi: 10.3390/foods11101388
70. Struyf, N, Van der Maelen, E, Hemdane, S, Verspreet, J, Verstrepen, KJ, and Courtin, CM. Bread dough and Baker’s yeast: an uplifting synergy. Compr Rev Food Sci Food Saf. (2017) 16:850–67. doi: 10.1111/1541-4337.12282
71. Ho, LH, Tan, TC, Abdul Aziz, NA, and Bhat, R. In vitro starch digestibility of bread with banana (Musa acuminata X balbisiana ABB cv Awak) pseudo-stem flour and hydrocolloids. Food Biosci. (2015) 12:10–7. doi: 10.1016/j.fbio.2015.07.003
72. Anton, AA, and Artfield, SD. Hydrocolloids in gluten-free breads: a review. Int J Food Sci Nutr. (2008) 59:11–23. doi: 10.1080/09637480701625630
73. Heiniö, RL, Noort, MWJ, Katina, K, Alam, SA, Sozer, N, de Kock, HL, et al. Sensory characteristics of wholegrain and bran-rich cereal foods – a review. Trends Food Sci Technol. (2016) 47:25–38. doi: 10.1016/j.tifs.2015.11.002
74. Moriartey, S, Temelli, F, and Vasanthan, T. Effect of health information on consumer acceptability of bread fortified with β-glucan and effect of fortification on bread quality. Cereal Chem. (2010) 87:428–33. doi: 10.1094/CCHEM-11-09-0146
75. Yassin, Z, Tan, YL, Srv, A, Monro, J, Matia-Merino, L, Lim, K, et al. Effects of xanthan gum, lambda-carrageenan and psyllium husk on the physical characteristics and Glycaemic potency of white bread. Foods. (2022) 11:1513. doi: 10.3390/foods11101513
76. Man, S, Adriana, P, Muste, S, Pop, A, and Andruta, M. Influence of psyllium husk (Plantago ovata) on bread quality. Bull Univ Agric Sci Vet Med Cluj Napoca Food Sci Technol. (2017) 74:33. doi: 10.15835/buasvmcn-fst:12631
77. León, AE, Ribotta, PD, Ausar, SF, Fernández, C, Landa, CA, and Beltramo, DM. Interactions of different carrageenan isoforms and flour components in Breadmaking. J Agric Food Chem. (2000) 48:2634–8. doi: 10.1021/jf991340a
80. Gómez, M, Ronda, F, Blanco, CA, Caballero, PA, and Apesteguía, A. Effect of dietary fibre on dough rheology and bread quality. Eur Food Res Technol. (2003) 216:51–6. doi: 10.1007/s00217-002-0632-9
81. Mandala, IG. Viscoelasticity: from theory to biological applications. InTech. England. Viscoelastic properties of starch and non-starch thickeners in simple mixtures or model food. BoD Books Demand. (2012) 36:217–36.
82. Fratelli, C, Santos, FG, Muniz, DG, Habu, S, Braga, ARC, and Capriles, VD. Psyllium improves the quality and shelf life of gluten-free bread. Foods. (2021) 10:954. doi: 10.3390/foods10050954
83. Surdyk, N, Rosén, J, Andersson, R, and Aman, P. Effects of asparagine, fructose, and baking conditions on acrylamide content in yeast-leavened wheat bread. J Agric Food Chem. (2004) 52:2047–51. doi: 10.1021/jf034999w
84. Dessev, T, Lalanne, V, Keramat, J, Jury, V, Prost, C, and Le-Bail, A. Influence of baking conditions on bread characteristics and acrylamide concentration. J Food Sci Nutr Res. (2020) 3:291–310.
85. Meybodi, NM, Mirmoghtadaie, L, Sheidaei, Z, and Mortazavian, AM. Wheat bread: potential approach to fortify its lysine content. Curr Nutr Food Sci. 15:630–7. doi: 10.2174/1573401315666190228125241
86. Ledl, F, and Schleicher, E. New aspects of the Maillard reaction in foods and in the human body. Angew Chem Int Ed Engl. (1990) 29:565–94. doi: 10.1002/anie.199005653
87. Granvogl, M, and Schieberle, P. Thermally generated 3-Aminopropionamide as a transient intermediate in the formation of acrylamide. J Agric Food Chem. (2006) 54:5933–8. doi: 10.1021/jf061150h
88. Hidalgo, FJ, Delgado, RM, Navarro, JL, and Zamora, R. Asparagine decarboxylation by lipid oxidation products in model systems. J Agric Food Chem. (2010) 58:10512–7. doi: 10.1021/jf102026c
89. Namir, M, Rabie, MA, Rabie, NA, and Ramadan, MF. Optimizing the addition of functional plant extracts and baking conditions to develop acrylamide-free Pita bread. J Food Prot. (2018) 81:1696–706. doi: 10.4315/0362-028X.JFP-18-150
92. Boyacı Gündüz, CP, and Cengiz, MF. Acrylamide contents of commonly consumed bread types in Turkey. Int J Food Prop. (2015) 18:833–41. doi: 10.1080/10942912.2013.877028
93. Li, L, Zhang, L, Zhang, T, Liu, Y, Lü, X, Kuipers, O, et al. (Meta)genomics-assisted screening of novel antibacterial lactic acid bacteria strains from traditional fermented milk from Western China and their bioprotective effects on cheese. Lebensm-Wiss-Technol. (2023) 175:114507
94. Protonotariou, S, Stergiou, P, Christaki, M, and Mandala, IG. Physical properties and sensory evaluation of bread containing micronized whole wheat flour. Food Chem. (2020) 318:126497. doi: 10.1016/j.foodchem.2020.126497
96. Claus, A, Mongili, M, Weisz, G, Schieber, A, and Carle, R. Impact of formulation and technological factors on the acrylamide content of wheat bread and bread rolls. J Cereal Sci. (2008) 47:546–54. doi: 10.1016/j.jcs.2007.06.011
97. Ahrné, L, Andersson, CG, Floberg, P, Rosén, J, and Lingnert, H. Effect of crust temperature and water content on acrylamide formation during baking of white bread: steam and falling temperature baking. LWT Food Sci Technol. (2007) 40:1708–15. doi: 10.1016/j.lwt.2007.01.010
98. Bråthen, E, and Knutsen, S. Effect of temperature and time on the formation of acrylamide in starch-based and cereal model systems, flat breads and bread. Food Chem. (2005) 92:693–700. doi: 10.1016/j.foodchem.2004.08.030
99. Mustafa, A, Andersson, R, Rosén, J, Kamal-Eldin, A, and Aman, P. Factors influencing acrylamide content and color in rye crisp bread. J Agric Food Chem. (2005) 53:5985–9. doi: 10.1021/jf050020q
100. Elmore, JS, Koutsidis, G, Dodson, AT, Mottram, DS, and Wedzicha, BL. Measurement of acrylamide and its precursors in potato, wheat, and rye model systems. J Agric Food Chem. (2005) 53:1286–93. doi: 10.1021/jf048557b
101. Taeymans, D, Wood, J, Ashby, P, Blank, I, Studer, A, Stadler, RH, et al. A review of acrylamide: an industry perspective on research, analysis, formation, and control. Crit Rev Food Sci Nutr. (2004) 44:323–47. doi: 10.1080/10408690490478082
102. Amrein, TM, Schönbächler, B, Escher, F, and Amado, R. Acrylamide in gingerbread: critical factors for formation and possible ways for reduction. J Agric Food Chem. (2004) 52:4282–8. doi: 10.1021/jf049648b
103. Hadiyanto, H, Asselman, A, van Straten, G, Boom, RM, Esveld, DC, and van Boxtel, AJB. Quality prediction of bakery products in the initial phase of process design. Innov Food Sci Emerg Technol. (2007) 8:285–98. doi: 10.1016/j.ifset.2007.01.006